
ARE YOU OF LEGAL VAPE AGE?
Please confirm that you are of legal age to purchase vaping products to access our site.

Please confirm that you are of legal age to purchase vaping products to access our site.
Some items are no longer available. Your cart has been updated.
This discount code cannot be used in conjunction with other promotional or discounted offer.
This article systematically explores the types, manufacturing technologies, and identification methods of vape. It begins by introducing the definition and development history of vape, followed by a detailed analysis of the characteristics of closed-system, open-system, and disposable vape. Next, it focuses on key manufacturing technologies, including atomizer technology, e-liquid formulations, and battery and control systems, while discussing identification research methods such as component analysis, performance testing, and safety evaluation. Finally, it summarizes the current development status of the e-cigarette industry and provides an outlook on its future trends. The article aims to provide theoretical foundations and technical support for the healthy development of the e-cigarette industry.
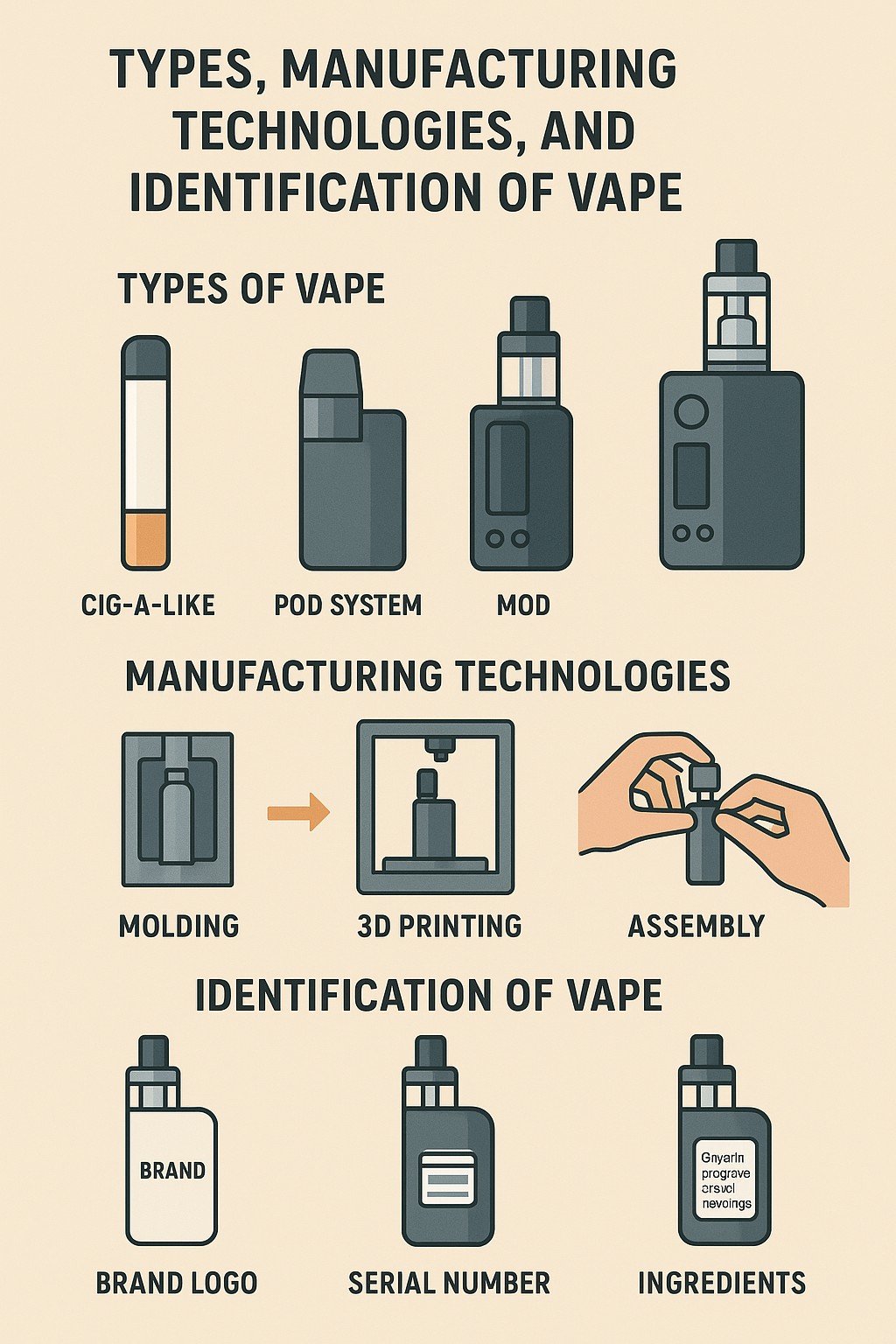
vape, as a novel nicotine delivery system, have rapidly gained popularity worldwide since their introduction in 2003. By heating a liquid containing nicotine to produce an aerosol for inhalation, vape may pose less harm to the human body compared to traditional cigarettes. However, the rapid and diversified development of vape has also introduced challenges, such as inconsistent product quality and safety concerns. In-depth research into the types, manufacturing technologies, and identification methods of vape holds significant importance. This article aims to systematically outline the types of vape, analyze their core manufacturing technologies, and explore effective identification research methods, thereby providing references for the standardized development and scientific regulation of the e-cigarette industry.
vape can generally be categorized by type into cartridges, devices, disposable vape, and kits. Cartridges, as an essential component of vape, serve as containers for storing e-liquid and are typically compact cylindrical structures made of plastic or metal. The e-liquid in cartridges is composed of propylene glycol, glycerin, flavorings, and nicotine (some without nicotine), with capacities generally ranging from 1 to 3 mL, varying by brand and model. Cartridges are convenient to use; they can be installed on compatible devices, and when the e-liquid is depleted, users simply replace them with new ones.
Devices are the core apparatus of vape, responsible for providing power to the cartridge and atomizing the e-liquid. They primarily consist of a battery, atomizer, and control circuit. Devices come in various designs, such as pen-style and box-style. Pen-style devices are compact, lightweight, and portable; box-style devices typically feature larger battery capacities and more advanced power adjustment functions to meet users' demands for vapor volume and flavor. The key performance of devices lies in battery endurance and atomization efficiency, with high-quality devices ensuring stable voltage output for thorough e-liquid atomization.
Disposable vape integrate the cartridge and device into a single, non-reusable product. They employ a pre-filled design with built-in e-liquid and battery, featuring a simple overall structure that requires no charging or cartridge replacement—users can simply pick them up and use them.
E-cigarette kits involve the bundled sale of devices, cartridges, and related accessories. Kits typically include the device, multiple cartridges, charging cables, and user manuals. The advantage of this product format is that it offers users a one-stop solution without the need to purchase devices and cartridges separately. Kit designs often emphasize compatibility and integration, ensuring optimal performance between the device and cartridges. Some branded kits feature unified aesthetic designs, with cartridge flavors harmoniously matched to the device's atomization effects.
Based on structure and functional characteristics, vape can primarily be divided into three categories: closed-system, open-system, and disposable.
Closed-system vape use pre-filled cartridges, allowing users to simply replace them for easy operation. These vape are typically compact and portable, though cartridge options are relatively limited. Representative products include JUUL and Vuse, which are popular for their ease of use and consistency. Closed-system designs prioritize user experience, with many incorporating smart technologies such as automatic power adjustment and dry-burn prevention.
Open-system vape allow users to add e-liquid themselves, offering greater customization. These systems usually require larger battery capacities and stronger atomization capabilities but involve more complex operations, necessitating some professional knowledge from users. Representative products include brands like GeekVape and SMOK. These products often provide various adjustable settings, such as power, temperature, and airflow control, to meet diverse user preferences. The key features of open systems are their flexibility and maintainability, enabling users to combine different atomizers and e-liquids according to their needs.
Disposable vape are pre-filled, non-reusable products that are compact, affordable, and convenient, suitable for temporary use or as entry-level options (see Figure 1). However, they have limited battery life and are not environmentally friendly. In recent years, with technological advancements, new types of vape have emerged, such as heat-not-burn tobacco products and nicotine salt vape. These products aim to meet user needs while exploring ways to reduce health risks. Heat-not-burn tobacco products release nicotine by heating rather than burning tobacco, theoretically reducing harmful substances. Nicotine salt vape use nicotine salts instead of freebase nicotine, providing a smoother inhalation experience and faster nicotine absorption.
Figure 1 Common disposable vape

VAPEPIE Max 40000 PUFFS disposable vape

VAPEPIE Ultra Phantom 30000 PUFFS

VAPEPIE Crystal Pop 15000 PUFFS
The core manufacturing technologies of vape primarily include atomizer technology, e-liquid formulations, and battery and control systems. The atomizer, as a key component, operates by using a heating element to convert e-liquid into an inhalable aerosol. Current mainstream atomizer technologies include ceramic cores, mesh cores, and cotton cores, each with unique advantages and limitations. For instance, ceramic cores are resistant to high temperatures and less prone to scorching; mesh cores provide larger heating surfaces and faster atomization speeds. Ceramic core atomizers are favored for their stable performance and longer lifespan; mesh core atomizers are praised for their rapid heating response and rich flavor profiles.
E-liquid formulations are decisive factors in the flavor and safety of vape. Typical e-liquid components include propylene glycol, vegetable glycerin, nicotine, and food-grade flavorings. The proportions and quality of these ingredients directly influence the user experience and potential risks. In recent years, the application of nicotine salt technology has enabled vape to deliver nicotine satisfaction closer to traditional cigarettes while reducing irritation. Nicotine salts are formed by combining freebase nicotine with organic acids, offering greater stability at low temperatures and a smoother inhalation experience. Additionally, e-liquids feature a wide variety of flavorings, ranging from traditional tobacco to fruits, desserts, and beverages. Non-tobacco flavored vape pose significant risks and appeal to minors due to their deceptive nature.
Battery and control systems determine the power output and safety of vape. Advanced vape are equipped with power regulation and temperature control functions to prevent overheating and the generation of harmful substances. Battery technology has evolved from early nickel-metal hydride batteries to modern lithium-ion batteries, which offer higher energy density and longer lifespans. In terms of control systems, many high-end vape use microprocessors for precise power and temperature regulation, along with safety protections such as short-circuit prevention, overcharge protection, and overheating safeguards. Furthermore, some vape include Bluetooth connectivity and smartphone apps, allowing users to customize settings and track usage data. The internal structure of e-cigarette devices is shown in Figure 2.
Figure 2 Internal structure of electronic cigarette smoking device

Research on e-cigarette identification primarily focuses on three aspects: component analysis, performance testing, and safety evaluation.
Component analysis aims to determine the chemical constituents in e-liquids and aerosols, including target components (such as nicotine) and potentially harmful substances (such as formaldehyde and acetaldehyde). Common analytical methods include gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS). These techniques enable accurate identification and quantification of various compounds in e-liquids and aerosols, providing reliable data for safety assessments. For example, GC-MS is used to detect volatile organic compounds in e-liquids, while LC-MS is more suitable for analyzing non-volatile components like nicotine and certain flavorings.
Performance testing evaluates indicators such as atomization efficiency, nicotine delivery efficiency, and battery performance, helping to understand the actual usage effects and consistency of vape. For instance, simulating real-world conditions can measure aerosol output and nicotine delivery at different power levels, assessing product stability. Performance tests include aerosol generation volume testing, nicotine delivery efficiency testing, and battery endurance testing, which not only support quality control but also inform product improvements. For example, optimizing atomizer designs and e-liquid formulations can enhance nicotine delivery efficiency, reducing overall aerosol inhalation by users.
Safety evaluation focuses on health risks associated with e-cigarette use, including acute toxicity and long-term effects. Cytotoxicity experiments and animal studies are common methods. Additionally, clinical studies are increasingly conducted to assess the actual impacts of vape on human health. Cytotoxicity experiments typically use human lung cells or other relevant cell lines, exposing them to e-cigarette aerosols to evaluate toxicity. Animal studies provide comprehensive toxicity data, including acute, subchronic, and reproductive toxicity. Clinical studies recruit volunteers to examine effects on the respiratory system, cardiovascular system, and nicotine dependency, which are essential for a thorough understanding of e-cigarette health risks.
Furthermore, the formal implementation of mandatory national standards for vape provides clear guidelines for production, sales, and regulation, establishing key points for identifying compliant products. In terms of packaging and appearance, product packaging must clearly indicate the manufacturer's name, address, contact information, product name, model, production date, shelf life, nicotine content, total nicotine amount, and standard code. Legitimate products often feature advanced anti-counterfeiting technologies, such as laser holographic labels and QR codes. Consumers or inspectors can verify authenticity by scanning codes or comparing anti-counterfeiting patterns. Some branded vape' QR codes reveal detailed traceability information, such as production batches and sales channels. From an overall appearance perspective, national standard products exhibit fine craftsmanship, high-quality shell materials without obvious scratches or burrs; cartridges and devices connect tightly with even gaps.
For component detection, focus on nicotine concentration and total amount, nicotine purity, additive usage, impurities and contaminants, and heavy metal content. For example, the national standard specifies a "white list" of 101 permitted additives. Using equipment like GC-MS, the types and contents of aerosol additives can be detected. If additives outside the list are found or usage exceeds maximum limits, the product is non-compliant.
Regarding performance indicators, e-cigarette devices and cartridges should have closed structures preventing manual refilling. Simulating real usage can check if they can be easily opened for filling. Additionally, test the sealing of devices and cartridges using e-liquid to observe for leaks. National standards require devices to have good sealing without leakage. To verify child-resistant activation features, check for specific button shapes or complex multi-step activation, along with protections against accidental startup, such as automatic locking after brief inactivity, requiring reactivation for reuse. Drop the e-cigarette from specified heights and observe for fire or explosion post-drop to test drop strength. In cases of battery failure causing internal pressure, check if the pressure relief direction aligns with the inhalation direction. National standards require the relief direction to differ from the inhalation direction for safety.
Using professional temperature measurement equipment, detect the atomization zone temperature. Waterproof performance must meet IPX4 protection requirements in GB/T 4208-2017 Chapter 6, preventing damage from water splashes in all directions. Under the national standard framework, identification and inspection should cover multiple dimensions, from packaging and appearance to components, performance, and safety, to accurately determine product compliance and protect consumer rights and market order.
The e-cigarette market has experienced rapid growth in recent years, particularly among younger demographics. According to market research data, the global e-cigarette market size is expected to continue expanding in the coming years. This growth is primarily driven by consumers' pursuit of healthier lifestyles and ongoing advancements in e-cigarette technology. However, rapid market expansion has also posed regulatory challenges, prompting governments worldwide to refine e-cigarette policies.
In the future, the e-cigarette industry will emphasize product safety and quality control. Innovations in atomization technology, safer e-liquid formulations, and intelligent control systems will become research priorities. For example, nanotechnology may be applied to atomizer designs to improve efficiency and reduce harmful substance generation. Additionally, the use of biodegradable materials will emerge as a key trend to mitigate environmental impacts. Meanwhile, interdisciplinary collaboration and long-term safety studies are crucial for comprehensively evaluating vape' potential effects. Only through scientific and standardized development can vape fulfill their potential as harm-reduction products and contribute to public health safety. Future research may focus more on long-term health impacts and their efficacy as smoking cessation aids.
vape, as a rapidly evolving new tobacco product, exhibit increasing diversity in types and continuous advancements in manufacturing technologies. Closed-system, open-system, and disposable vape each have unique features to meet varied user needs. Innovations in atomizer technology, e-liquid formulations, and battery control systems drive ongoing performance improvements. However, e-cigarette safety remains a persistent concern. Through identification research methods such as component analysis, performance testing, and safety evaluation, a better understanding of e-cigarette characteristics can be achieved, providing scientific bases for developing relevant standards and regulatory policies.

Comment