
ARE YOU OF LEGAL VAPE AGE?
Please confirm that you are of legal age to purchase vaping products to access our site.

Please confirm that you are of legal age to purchase vaping products to access our site.
Some items are no longer available. Your cart has been updated.
This discount code cannot be used in conjunction with other promotional or discounted offer.
As awareness grows regarding the chronic diseases associated with traditional cigarette smoking, and with global smoking rates remaining high, electronic cigarettes (Vapes) have emerged as a popular aid for smoking cessation. Supported by government initiatives in several European countries to promote Vapes for quitting smoking, the Vape market has experienced rapid innovation and expansion. As a major producer and exporter of Vapes, China has introduced numerous regulations in recent years to standardize manufacturing and usage, fostering sustainable industry growth. Concurrently, the transportation hazards of Vapes have attracted widespread domestic and international attention. The flammability risk of e-liquid can be assessed via its flash point. This study investigates the influence of ethanol content on the flammability of an e-liquid model through flash point determination experiments. Results indicate that the flash point of the e-liquid decreases with increasing ethanol content, providing valuable reference for predicting the flammability risks of e-liquids with known ethanol concentrations.
According to the national standard Electronic Cigarettes (GB 41700-2022), Vape liquid is defined as a liquid aerosol, which consists of mixtures and auxiliary substances that can be fully or partially atomized into an inhalable aerosol by an electronic device. Vape liquid is heated and atomized via an Vape atomizer to produce a mist resembling cigarette smoke. The primary base components of most e-liquids are food-grade glycerol (commonly known as glycerin) and 1,2-propanediol (propylene glycol), which together can account for up to 90% of the e-liquid by mass percentage.
The standard also specifies that only 101 additives may be incorporated into the aerosol, with common ones including ethanol and ethyl acetate. Adding ethanol to e-liquid facilitates atomization and enhances the cooling sensation, improving mouthfeel. However, ethanol is classified as a Category 3 flammable liquid, and its inclusion in e-liquid may impart flammability to the product.
The flash point, also known as the ignition point, is the lowest temperature at which a material or product forms a vapor-air mixture that ignites upon contact with a flame and burns immediately. It reflects the material's evaporation tendency and thermal stability. Per the United Nations Recommendations on the Transport of Dangerous Goods: Model Regulations, liquids with a closed-cup flash point not exceeding 60°C are classified as flammable. The flash point of 1,2-propanediol is 99.0°C, that of glycerol is 160.0°C, and that of ethanol is 14.0°C [6].
Current research primarily focuses on the flash point of ethanol-water solutions, with limited studies on ethanol in organic solvent mixtures. This article, based on the United Nations Manual of Tests and Criteria, uses a mixture of glycerol and 1,2-propanediol as the e-liquid base model and ethanol as an additive to examine the impact of ethanol on the flash point of the e-liquid model [4].
1.1 Instruments
SETAFLASH 82160-2U Automatic Flash Point Tester (SETA, UK); XSR105DU Analytical Balance (Mettler-Toledo).
1.2 Reagents
Anhydrous ethanol (AR grade); Propylene glycol (AR grade); Glycerol (AR grade).
1.3 Procedures
1.3.1 Solution Preparation
Using an analytical balance, weigh ethanol, 1,2-propanediol, and glycerol in volumetric flasks and mix thoroughly to obtain ethanol-propylene glycol-glycerol mixtures at various mass fractions.
1.3.2 Flash Point Determination
The flash point testing method follows ISO 3679:2022 Determination of flash point - Method for flash no-flash and flash point by small scale closed cup tester.
Preliminary flash point testing: Conduct initial tests approximately 10°C below the expected flash point [7]. If ignition occurs, reduce the temperature by 5°C and retest with a fresh sample. If no ignition occurs, increase the temperature by 5°C and retest. The lowest temperature at which ignition occurs, in 5°C intervals, is the preliminary flash point [2].
Formal flash point testing: Set the test temperature 2°C below the preliminary flash point and observe for flame ignition. If ignition occurs, decrease the temperature by 1°C and retest with a fresh sample. If no ignition occurs, increase by 1°C and retest. The lowest temperature at which ignition occurs is the sample's flash point [3].
2.1.1 Ethanol-Propylene Glycol and Glycerol Mixtures
This experiment aims to investigate the impact of ethanol addition on the flash point of propylene glycol-glycerol mixtures compared to water, and the effect of propylene glycol-to-glycerol ratios on the flash point when ethanol content is constant.
From the experimental data in Tables 1 and 2, at 5% ethanol content, the flash point of propylene glycol-glycerol mixtures is lower than that of aqueous solutions. This may be attributed to the polar nature of ethanol and water, following the principle of like dissolves like, and the presence of hydrogen bonding in water, which hinders ethanol volatilization, resulting in a higher flash point for ethanol-water solutions [6].
The data from Table 3 were organized and analyzed, as shown in Table 4 and Figure 1.
Table 1 Closed-cup flash point data of 5%ethanol solution
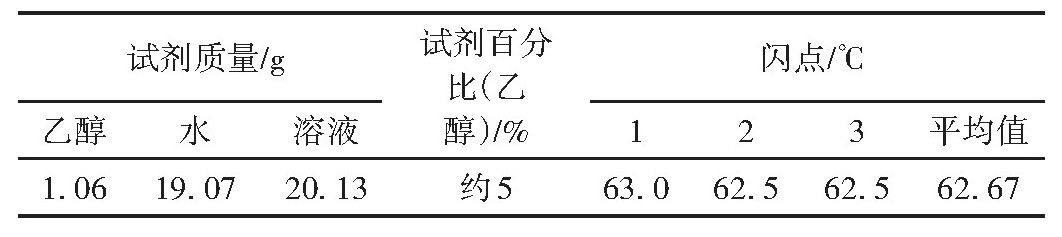
Table 2 Closed-cup flash point data of 5%ethanol in propanediol and glycerol (1:1) mixed solution

Table 3 Closed-cup flash point data of 5%ethanol in propanediol and glycerol mixed solution of different ratio
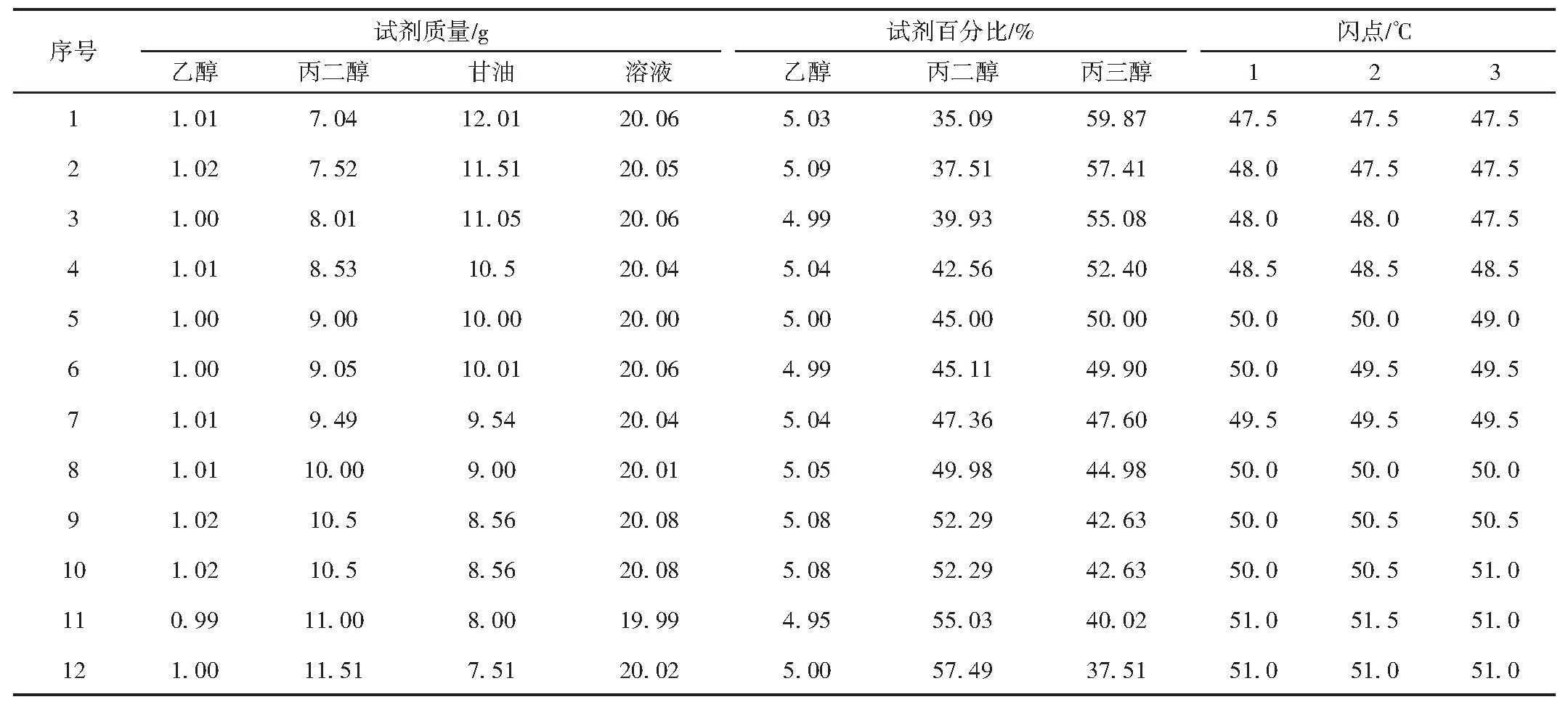
Table 4 Closed-cup flash point data collation of 5%ethanol in propanediol and glycerol mixed solution of different
ratio
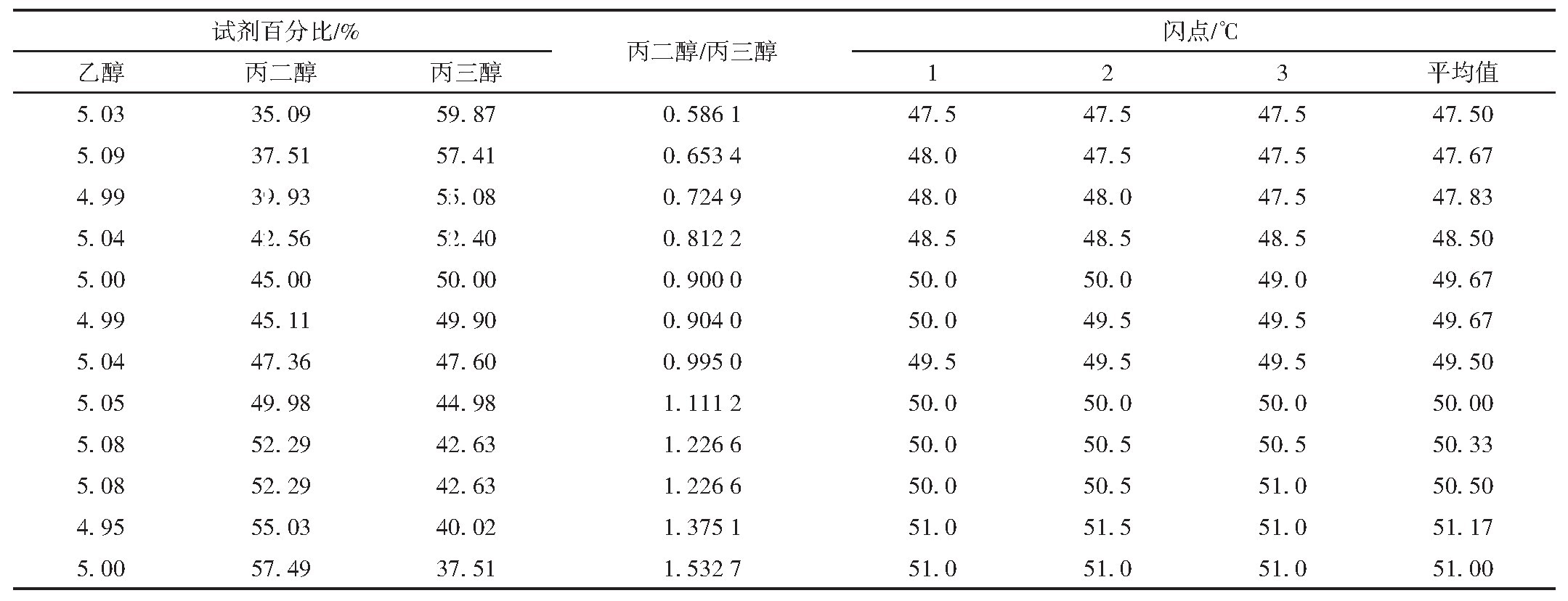
Fig.1 Trend of flash point of 5%ethanol in propanediol and glycerol mixed solution of different ratio
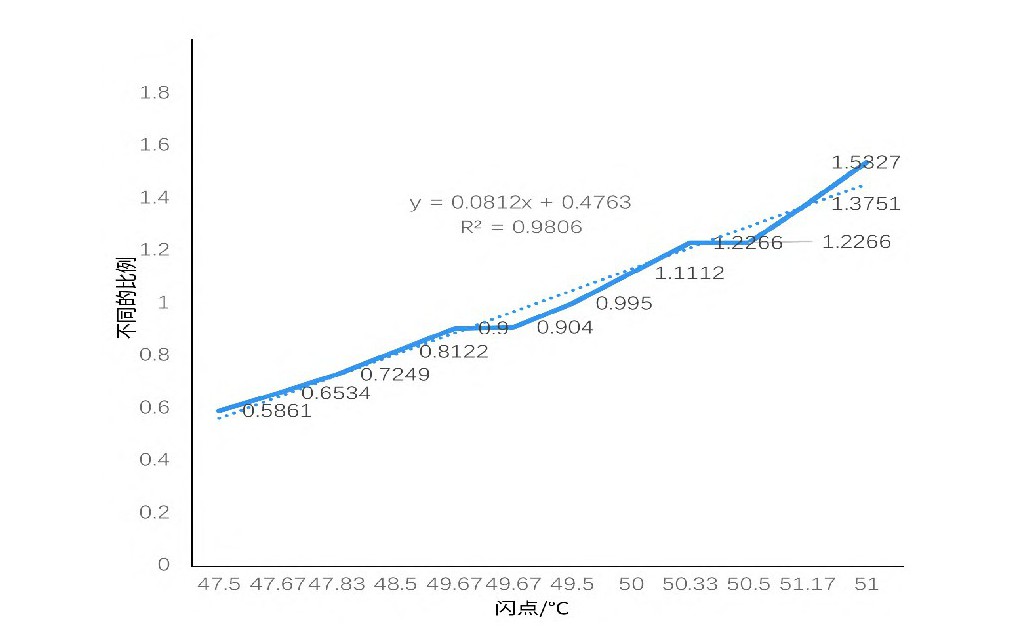
Fig.2 Trend of flash point of ethanol of different ratio in propanediol and glycerol (1:1) mixed solution
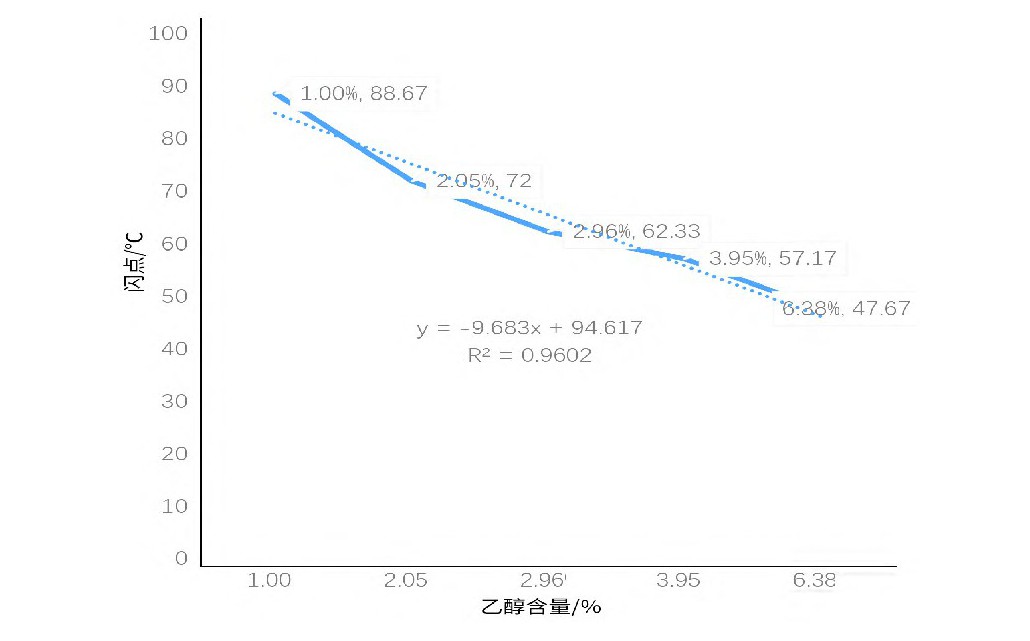
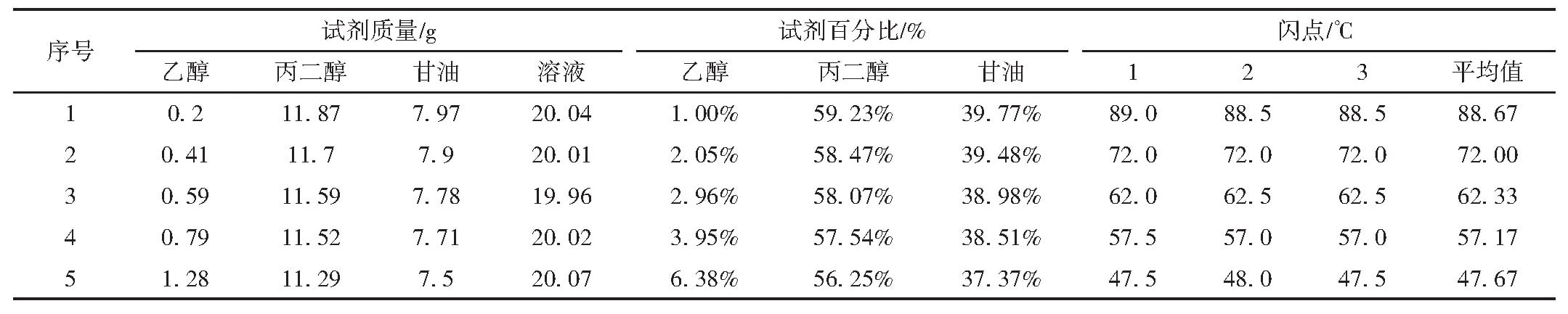
Table 5 Closed-cup flash point data of ethanol of different ratio in propanediol and glycerol (1:1) mixed solution
From Table 4 and Figure 1, when ethanol content is fixed, the mixture's flash point decreases with increasing propylene glycol content. Generally, as the proportion of the lower flash point component increases, the mixture's flash point decreases; thus, reducing the higher flash point glycerol and increasing the lower flash point propylene glycol lowers the overall flash point.
Based on preliminary results, this study selected an equal ratio of propylene glycol and glycerol as the e-liquid base model, varying ethanol content to determine the final mixture's flash point and explore the relationship between e-liquid flash point and ethanol content [5].
From Table 5 and Figure 2, with a fixed propylene glycol-to-glycerol ratio, higher ethanol content correlates with a lower flash point. At approximately 3% ethanol, the flash point is around 60°C, near the threshold for flammable liquid classification.
Experiments reveal that ethanol has a greater impact on the flash point of propylene glycol-glycerol mixtures compared to water, with the flash point decreasing as ethanol content increases. Although actual e-liquids have more complex compositions, where interactions among multiple substances may influence ethanol's effect on flash point, additives are typically present in low concentrations with minimal impact. Combining these findings, it can be predicted that e-liquids with ethanol content exceeding 3% may have flash points below 60°C, necessitating consideration of flammability risks during transportation.

Comment